This is an age of science and it has changed everything under the sun. In the past, people could only dream of things like flying in the sky and contacting others from long distances but now all these things can actually happen. We can fly in air by sitting in air planes. We can travel long distances in our cars and this has converted the distances of days into hours. We can contact each other with the help of cell phones and now the technology of the smart phone has revolutionized the world.
Today, we are living in the world which is full of wonders of science. These all technological advancements have actually made our lives very easy. We cannot live a single day without these inventions as we are so much dependent upon them. Man is always in the urge of finding and getting more. This urge keeps him motivated for finding and researching new things. Energy is the basic thing to live a life on this planet. We need energy for almost anything in our life. There are many forms of energy like the mechanical, electrical and the biological energy. When it comes to the electrical and the mechanical energy the man has always tried to find new ways of acquiring this energy. The urge of finding and capturing a new mode of energy made him to discover the solar cells, the cells which take up their energy from the sun and converts it into the mechanical energy to run many appliances. One of such solar cell is called as the perovskite solar cell.
The perovskite solar cell is a special kind of solar cell in which there is present a perovskite absorber, it is a hybrid organic-inorganic lead or tin halide-based stuff, and it is responsible for the production of electricity from sunlight. The name of the perovskite solar cell is obtained from the ABX3 crystal structure of the absorber material. The most common perovskite absorber which is studied is the methyl ammonium lead tri-halide (CH3NH3PbX3), and its band gap is between 2.3 eV and 1.57 eV but it entirely depends upon the halide content. Formamidinum lead tri-halide whose band gap is between 2.23 and 1.43 eV is a new studied material which shows the credibility. It is capable of higher efficiencies. The inclusion of the lead is a common concern in the perovskite solar cell. This is one of the main components of the perovskite solar cell. This concern has been briefed by Noel et al with the introduction of a perovskite absorber which is made of the tin. In this perovskite solar cell the lead is fully replaced with tin, which yields a power-conversion efficiency of more than 6%.
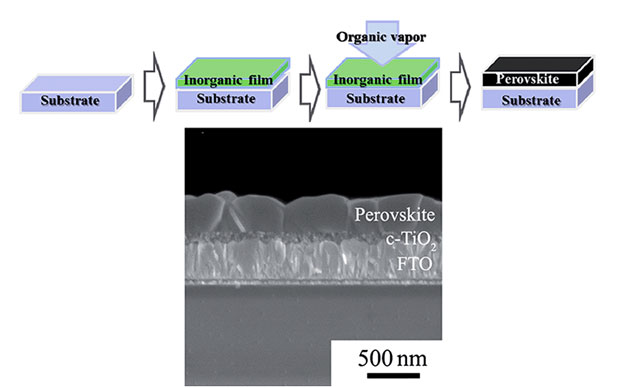
These solar cells function efficiently in a number of different architectures. The function entirely depends either on the role of the perovskite material that is used in the device, or the nature of the top and bottom electrode that are supplied in the cell. The solar cell devices in which the positive charges are extracted by the electrode present at the bottom, anode, can be surely divided into 'sensitized', where the function of perovskite is mainly to act as a light absorber. There is also another different class of architectures. In this class of the architecture the transparent electrode present at the bottom acts as cathode by collecting the photo generated p-type charge carriers.
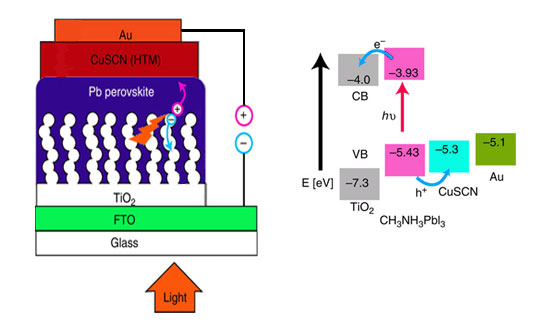
Due to the rapid improvements of the perovskite solar cell in a very short amount of time, the perovskite solar cell has become one of the most important and promising photo voltaic cells of the century. At present the efficiency of these cells is 15% but this efficiency is expected to be improved further. In this way, they would be used in number of ways with greater efficiency. The use of the perovskite solar cell is important because of the following reason:
Toxicity: The perovskite solar cells are now made of the tin. Lead is a very pollutant material and is responsible for the bad health of the humans. The use of tin also promises a great efficiency up to 6%.
Easy fabrication: The first and the previous solar cells used complex fabrication methods. At an experiment conducted last year by Snaith at Oxford claimed that they made a cell with 15% efficiency. These cells were made with vapor deposition which is regarded as the standard method in the semiconductor industry. Now the Mei’s lab has gone one step further and has made a cell using drop casting method.
Durability: The perovskite cells made up of tin are also very durable as compared to the other cells. In an experiment conducted by Mei they just achieved a 1000 hours exposure without any degradation.
The major disadvantage is the use of the liquid electrolyte. This liquid electrolyte has the temperature stability problems. The electrolyte may freeze at lower temperature and may expand at higher temperature. The other problem is that many expensive compounds are needed to make this solar cell. The third problem associated with it is that the compounds are highly volatile and should be properly sealed otherwise they may prove deadly to the human health.
Stability: One of the big challenges for perovskite solar cells is the issue of the stability both in terms of long term and short term. The organic constituent of the absorber material are made of the highly water soluble material which in moist environment makes the devices highly prone to rapid degradation. The use of the absorber with a composite of the carbon nanotubes and an inert polymer matrix are successfully used to prevent the absorber from the degeneration.
The perovskite solar cells have greater amount of efficiency, 15%, as compared to other cells. This efficiency and the stability of the perovskite can be improved with the use of the solid-hole conductor.